Background
Renal impairment is a life threatening complication of myeloma with up to 20-25% of patients presenting with renal dysfunction. Around 28% of newly diagnosed myeloma patients with renal failure do not survive beyond 100 days compared with 10% overall. In addition to poor survival, there are no quality of life (QoL) data from published renal failure myeloma studies to guide management. OPTIMAL trial utilizes the EuroQoL (EQ-5D-3L) heath questionnaire, a validated instrument for health outcomes, to measure QoL in these patients. It is a 3 level, 5 dimensional questionnaire, assessing mobility, self-care, usual activity, pain/discomfort and anxiety/depression with either no problems, some problems or extreme problems. Participants also rate their current health on a visual analogue scale (VAS) of 0-100. We hypothesize that reported QoL in typical myeloma trials are better than the QoL for patients with renal impairment such as those recruited into the OPTIMAL trial.
Methods
OPTIMAL is a randomised, multi-centre phase II trial of newly diagnosed myeloma patients with renal impairment (ClinicalTrials.gov Identifier: NCT02424851). Participants were randomised to receive four 12 week cycles of Bortezomib, Bendamustine and Dexamethasone (BBD) or Thalidomide, Bendamustine and Dexamethasone (BTD); all participants received bendamustine and dexamethasone in 3 week cycles. Participants not considered suitable for autologous stem cell transplant (ASCT) could be given a further 2 cycles of treatment in their respective arms.
Patient reported outcomes were measured using the validated EQ-5D-3L. Participants were asked to complete a questionnaire at baseline and on day 1 of each treatment cycle and at 1 month and 12 month follow up visits. Differences in changes of EQ-5D-3L and the VAS scores between baseline and 1 month follow up were assessed by 2 sample t-tests.
To compare the EQ-5D-3L data from the OPTIMAL trial to others, a literature search was conducted to see if any data were available that provided further insight into the QoL in patient's diagnosed with myeloma and renal failure. PUBMED and MEDLINE were explored and authors emailed if EQ-5D-3L data was not identified.
Results
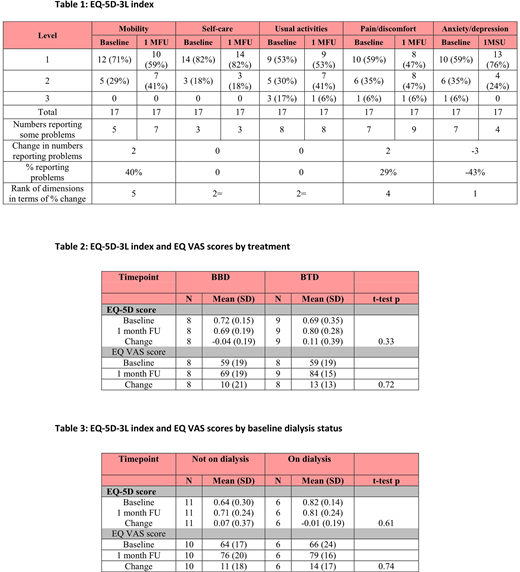
OPTIMAL recruited 31 patients between March 2015 and March 2019 from 7 centres within the UK. Of these, 17 patients completed EQ-5D-3L at baseline and at 1 month follow up; 8 on BBD and 9 on BTD. Sixty five per cent of patients were ≤70 years old, 76% male, 35% were CKD stage 4 and 65% were CKD stage 5, 59% had planned ASCT, 88% had ECOG performance status 0 or 1, 35% were on dialysis and 94% were ISS stage III. At baseline a mean of 35.2% of patients reported some or extreme problems across the 5 QoL domains compared with 36.6% at 1 month follow up (Table 1). No significant changes were detected in mean EQ-5D-3L (p=0.33) or VAS scores (p=0.72) between baseline and 1 month follow up by treatment arm (Table 2). Additionally, no differences in EQ-5D-3L scores were found between age group or dialysis status (p>0.1; Table 3). However, some improvement in anxiety and depression levels were observed overall with 59% of patients reporting none at baseline increasing to 76% at follow up. No changes were reported in self-care or the ability to perform usual activities. Mobility decreased from 71% experiencing no problems to 59%.
In order to compare QoL reported by OPTIMAL with QoL reported in published myeloma trials, a literature search was undertaken and identified 48 articles of which only 11 studies reported QoL and only 4 of these reported the EQ-5D-5L.
No studies assessed QoL in patients similar to the OPTIMAL trial patient population with renal impairment. However a couple of studies demonstrated improved QoL at 3 months compared to baseline across treatment arms.
Conclusion
It is well known that treatments used within clinical trials can significantly improve the outcomes for patients but it is important to also measure the impact on patient's QoL. The OPTIMAL trial demonstrated that this poor prognostic set of patients with renal impairment had significant reduction in anxiety and depression at 1 month follow up. It was disappointing that a literature search demonstrated a paucity of QoL data routinely collected and reported within clinical trials. Patients need to know the impact of treatments on outcomes such as survival but also need to know the impact on their QoL.
Funding: NAPP Pharmaceuticals, JANSSEN-Cilag Ltd and Blood Cancers UK.
Ramasamy:Takeda: Research Funding; Sanofi: Membership on an entity's Board of Directors or advisory committees; Takeda: Honoraria; Bristol Myers Squibb: Research Funding; GSK: Membership on an entity's Board of Directors or advisory committees; Bristol Myers squibb: Membership on an entity's Board of Directors or advisory committees; Janssen: Membership on an entity's Board of Directors or advisory committees; Oncopeptides: Honoraria; Janssen: Research Funding; Amgen: Honoraria; Sanofi: Honoraria; Abbvie: Membership on an entity's Board of Directors or advisory committees; Takeda: Membership on an entity's Board of Directors or advisory committees; Amgen: Research Funding; Janssen: Honoraria; Amgen: Membership on an entity's Board of Directors or advisory committees; Bristol Myers Squibb: Honoraria; Oncopeptides: Membership on an entity's Board of Directors or advisory committees; Takeda: Speakers Bureau. Lindsay:Amgen: Other: Travel Expenses; Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel Expenses; Takeda: Honoraria, Other: Travel Expenses.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal